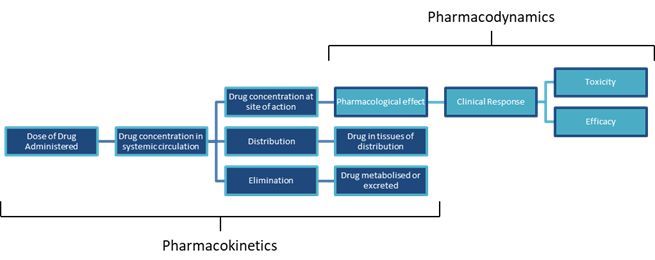
Pharmacokinetics/Toxicokinetics
Primary Objective
To describe systemic exposure achieved in animals and relationship to dose level, time course of toxicity study and gender differences.
Secondary Objectives
To related exposure achieved to toxicological findings and contribute to the assessment of the relevance of these findgins to clinical safety.
To provide information which, in conjunction with the toxicity findings, contributes to the design of subsequent non-clincal toxicity studies.